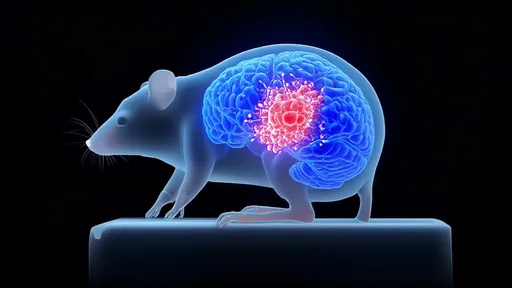
The human brain, with its intricate network of neurons and blood vessels, has long been a subject of fascination and mystery. Among its many enigmas, glioblastoma—the most aggressive form of brain cancer—remains a formidable challenge for researchers and clinicians alike. Traditional methods of studying these tumors often rely on post-mortem examinations or invasive biopsies, which provide limited insights into the dynamic behavior of cancer cells in a living organism. However, a groundbreaking technique known as the transparent skull window is revolutionizing our ability to observe gliomas in real time, offering unprecedented opportunities for understanding their progression and treatment.
Developed by a team of neuroscientists and bioengineers, the transparent skull window involves replacing a portion of the skull with a durable, optically clear material. This innovative approach allows researchers to peer directly into the brain using advanced imaging technologies, such as two-photon microscopy. Unlike traditional methods that require sacrificing the animal model or performing repetitive surgeries, this technique enables longitudinal studies, where the same tumor can be monitored over weeks or even months. The implications are profound: scientists can now track how glioma cells invade healthy tissue, respond to therapies, and evolve resistance—all within a living brain.
One of the most striking revelations from these live observations is the heterogeneous nature of gliomas. Tumors are not uniform masses but rather collections of cells with varying behaviors and genetic profiles. Some cells migrate rapidly along blood vessels, while others remain dormant, evading detection and treatment. This heterogeneity has long been suspected but has never been visualized with such clarity. The transparent skull window has also shed light on the tumor microenvironment, revealing how immune cells interact—or fail to interact—with cancer cells. These findings are reshaping our understanding of why gliomas are so resistant to conventional therapies.
Beyond basic research, the transparent skull window holds immense promise for translational medicine. By observing how tumors respond to experimental drugs in real time, researchers can identify promising compounds more efficiently. For instance, a drug that appears to shrink the tumor in a petri dish might fail to penetrate the blood-brain barrier or trigger unexpected resistance mechanisms in vivo. The ability to witness these dynamics firsthand accelerates the drug development process, potentially bringing life-saving treatments to patients faster. Moreover, the technique could pave the way for personalized medicine, where therapies are tailored based on how an individual's tumor behaves under observation.
Despite its potential, the transparent skull window is not without challenges. The procedure requires meticulous surgical precision to avoid damaging the underlying brain tissue, and the artificial window must remain stable for extended periods. There are also limitations in imaging depth, as current technologies cannot visualize structures far beneath the brain's surface. Nevertheless, ongoing advancements in materials science and microscopy are steadily overcoming these hurdles. Some researchers are even exploring the integration of nanotechnology, such as fluorescent probes, to enhance the visibility of specific cell types or molecular processes.
Ethical considerations also come into play, particularly as the technique moves closer to potential human applications. While the transparent skull window has so far been used only in animal models, its success raises questions about whether a similar approach could one day benefit glioma patients. Imagine a future where neurosurgeons could monitor tumor recurrence or treatment response without repeated invasive procedures. Though such scenarios remain speculative, they underscore the transformative potential of this technology.
In the broader context of cancer research, the transparent skull window exemplifies a paradigm shift toward dynamic, live-cell imaging. It complements other cutting-edge tools like organoids and liquid biopsies, collectively moving the field away from static snapshots and toward a more holistic understanding of disease progression. As these technologies converge, they offer hope not just for gliomas but for a wide range of cancers that have eluded effective treatment.
The journey from laboratory discovery to clinical application is often long and fraught with setbacks, but the transparent skull window represents a beacon of progress. By illuminating the darkest corners of glioma biology, it brings us one step closer to outsmarting a disease that has defied cure for decades. For patients and their families, that glimmer of hope—visible now through a literal window into the brain—is more valuable than ever.

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025